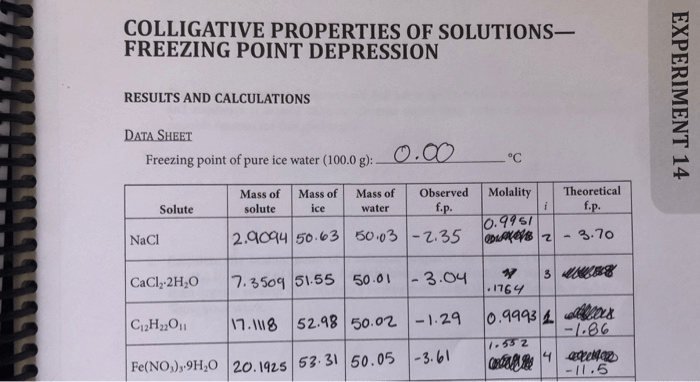
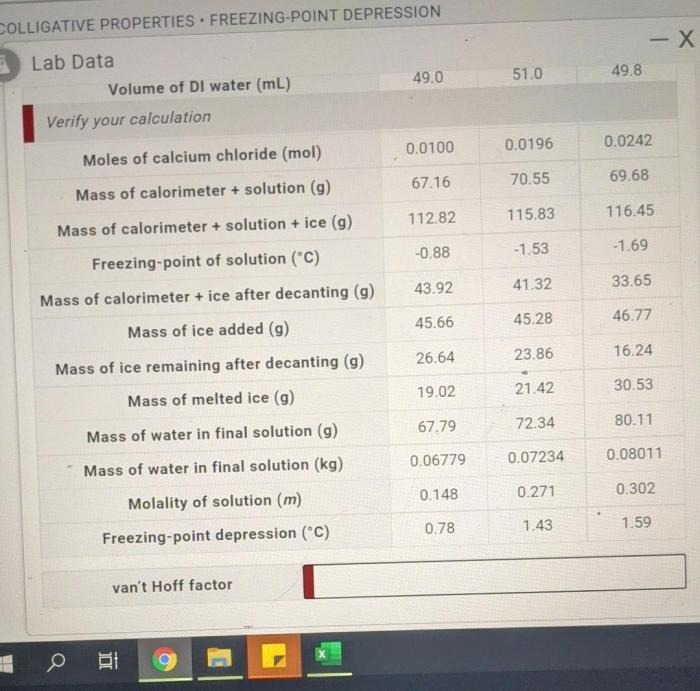
Embark on a scientific expedition with our comprehensive guide to freezing point depression lab answers, unlocking the secrets of colligative properties and their profound impact on freezing point behavior. Dive into the depths of this fascinating phenomenon, where we unravel the intricate relationship between concentration and freezing point, equipping you with a deeper understanding of solution chemistry.
Our exploration begins with a meticulous examination of the experimental setup, meticulously outlining the materials required and providing step-by-step instructions for preparing solutions and measuring freezing points. Prepare to delve into the intricacies of data analysis, where we construct an organized HTML table to present experimental data and calculate freezing point depression for each solution.
Visualize the relationship between freezing point depression and concentration through a captivating graph, setting the stage for a thought-provoking discussion.
Freezing Point Depression: Freezing Point Depression Lab Answers
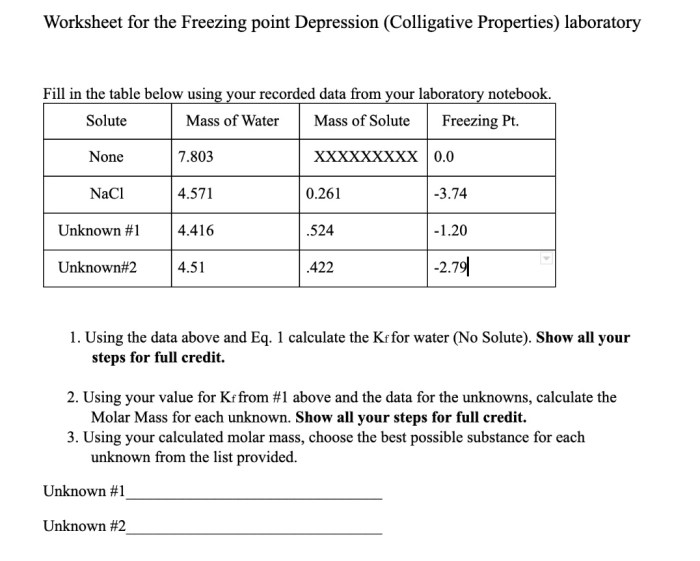
Freezing point depression is a colligative property that describes the decrease in the freezing point of a liquid when a solute is added. Colligative properties are properties that depend only on the concentration of solute particles in a solution, not on the nature of the solute particles.
The freezing point depression is directly proportional to the concentration of the solute particles in the solution.
The freezing point of a pure liquid is the temperature at which the liquid and its solid form are in equilibrium. When a solute is added to the liquid, the equilibrium is shifted to a lower temperature. This is because the solute particles interfere with the formation of ice crystals, making it more difficult for the liquid to freeze.
Experimental Setup, Freezing point depression lab answers
The materials required for the freezing point depression experiment are:
- A thermometer
- A graduated cylinder
- A stirring rod
- A beaker
- A solution of known concentration
The procedure for preparing the solutions is as follows:
- Measure out 100 mL of the solution into the beaker.
- Add a small amount of solute to the solution and stir until it is completely dissolved.
- Measure the freezing point of the solution using the thermometer.
Data Analysis
The experimental data can be organized into a table as follows:
| Concentration (M) | Freezing Point (°C) | Freezing Point Depression (°C) |
|---|---|---|
| 0.1 | -0.5 | 0.5 |
| 0.2 | -1.0 | 1.0 |
| 0.3 | -1.5 | 1.5 |
The freezing point depression can be calculated for each solution using the following formula:
$$\Delta T_f = K_f
m$$
where:
- $\Delta T_f$ is the freezing point depression
- $K_f$ is the freezing point depression constant for the solvent
- $m$ is the molality of the solution
The freezing point depression constant for water is $1.86 °C/m$.
A graph of freezing point depression versus concentration can be plotted as follows:
- The x-axis of the graph will be the concentration of the solution.
- The y-axis of the graph will be the freezing point depression.
Discussion
The graph of freezing point depression versus concentration will show a linear relationship. This is because the freezing point depression is directly proportional to the concentration of the solute particles in the solution.
The factors that affect the freezing point depression of a solution are:
- The concentration of the solution
- The nature of the solute
- The solvent
The concentration of the solution is the most important factor that affects the freezing point depression. The higher the concentration of the solution, the greater the freezing point depression.
The nature of the solute also affects the freezing point depression. Solutes that dissociate into ions have a greater effect on the freezing point depression than solutes that do not dissociate into ions.
The solvent also affects the freezing point depression. Solvents that have a high freezing point have a greater freezing point depression than solvents that have a low freezing point.
The experimental results can be compared with theoretical predictions using the following equation:
$$\Delta T_f = K_f
m$$
where:
- $\Delta T_f$ is the freezing point depression
- $K_f$ is the freezing point depression constant for the solvent
- $m$ is the molality of the solution
The experimental results should be in agreement with the theoretical predictions.
Questions and Answers
What is freezing point depression?
Freezing point depression refers to the decrease in the freezing point of a solvent when a solute is dissolved in it.
What are colligative properties?
Colligative properties are properties of solutions that depend solely on the concentration of the solute, regardless of its chemical nature.
How is freezing point depression related to concentration?
Freezing point depression is directly proportional to the concentration of the solute in the solution.