Complete an orbital diagram for boron. – Complete an orbital diagram for boron, an enthralling journey into the realm of quantum mechanics, where we unravel the intricate dance of electrons within the atom’s orbitals. This guide delves into the fundamental principles governing electron distribution, offering a clear understanding of boron’s atomic structure and its implications in chemical bonding.
Boron, with its unique atomic number and electron configuration, serves as an ideal subject for exploring the principles of orbital diagrams. We embark on a detailed examination of its s, p, and d orbitals, deciphering the rules that dictate electron distribution and the significance of valence electrons in shaping boron’s chemical behavior.
Boron’s Atomic Structure
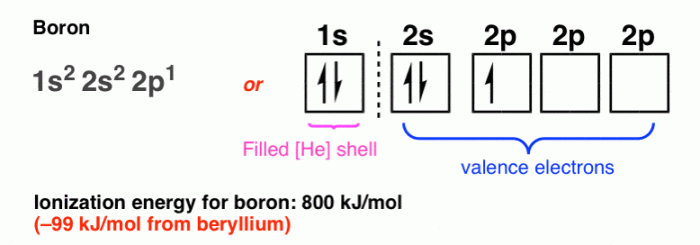
Boron, an essential element in many chemical processes, has an atomic number of 5, indicating the presence of 5 protons in its nucleus. The electron configuration of boron is 1s 22s 22p 1, which means it has two electrons in the first energy level, two electrons in the second energy level, and one electron in the third energy level.
The valence electrons, which are the electrons in the outermost energy level, play a crucial role in determining the chemical properties of boron. Boron has three valence electrons, making it a trivalent element.
Orbital Diagram Representation: Complete An Orbital Diagram For Boron.
| Orbital | Shape | Number of Electrons | Spin |
|---|---|---|---|
| 1s | Spherical | 2 | Paired |
| 2s | Spherical | 2 | Paired |
| 2px | Dumbbell | 1 | Unpaired |
The orbital diagram shows that boron has two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p xorbital. The 2p yand 2p zorbitals are empty.
Electron Distribution
The distribution of electrons in orbitals follows specific rules:
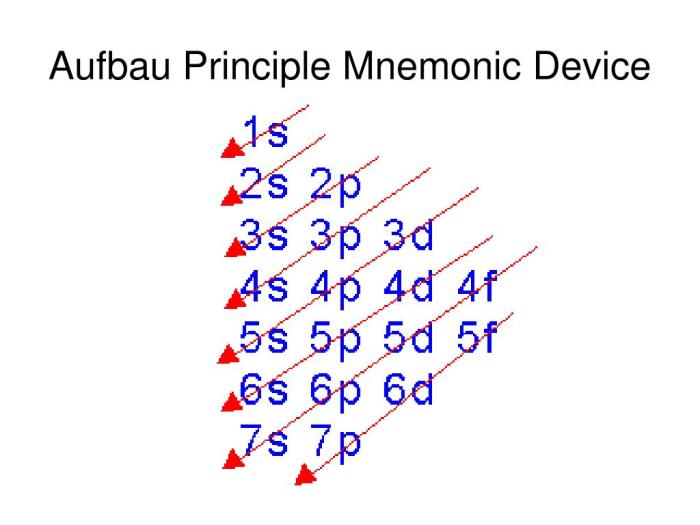
- Aufbau principle:Electrons occupy the lowest energy orbitals first.
- Hund’s rule:When multiple orbitals of the same energy are available, electrons occupy them singly before pairing up.
According to the Aufbau principle, the 1s orbital is filled first, followed by the 2s orbital, and then the 2p orbitals. Hund’s rule dictates that the single electron in the 2p orbital occupies the 2p xorbital before pairing up with another electron in the 2p yor 2p zorbitals.
Valence Electrons and Bonding
Valence electrons are responsible for chemical bonding. Boron’s three valence electrons allow it to form covalent bonds with other atoms.
In a covalent bond, two atoms share a pair of electrons. Boron can form single, double, or triple bonds depending on the number of valence electrons available from the other atom.
For example, boron forms a single covalent bond with hydrogen in the compound BH 3, a double covalent bond with oxygen in the compound B 2O 3, and a triple covalent bond with nitrogen in the compound BN.
FAQ Insights
What is the significance of boron’s valence electrons?
Boron’s valence electrons play a crucial role in determining its chemical properties. With three valence electrons, boron can participate in covalent bonding, forming stable compounds with other elements.
How does the Aufbau principle apply to boron’s electron configuration?
The Aufbau principle dictates that electrons fill atomic orbitals in order of increasing energy levels. For boron, the Aufbau principle predicts the electron configuration of 1s²2s²2p¹.