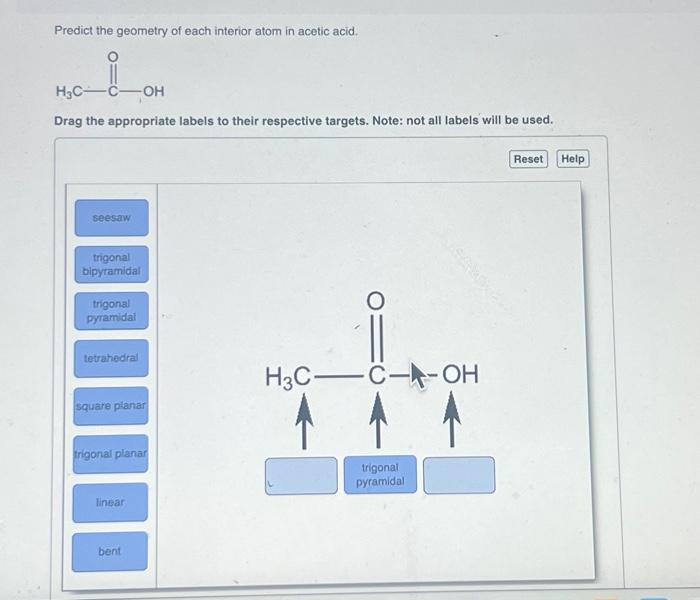
Predict the geometry of each interior atom in acetic acid. – Predicting the geometry of each interior atom in acetic acid provides a comprehensive understanding of its molecular structure and behavior. This article explores the principles and methods used to determine the geometry of these atoms, offering insights into their chemical bonding and reactivity.
The molecular formula of acetic acid, CH3COOH, reveals a central carbon atom surrounded by three other atoms: two oxygen atoms and a hydrogen atom. The hybridization of the central carbon atom plays a crucial role in determining the geometry around it.
Structural Features of Acetic Acid
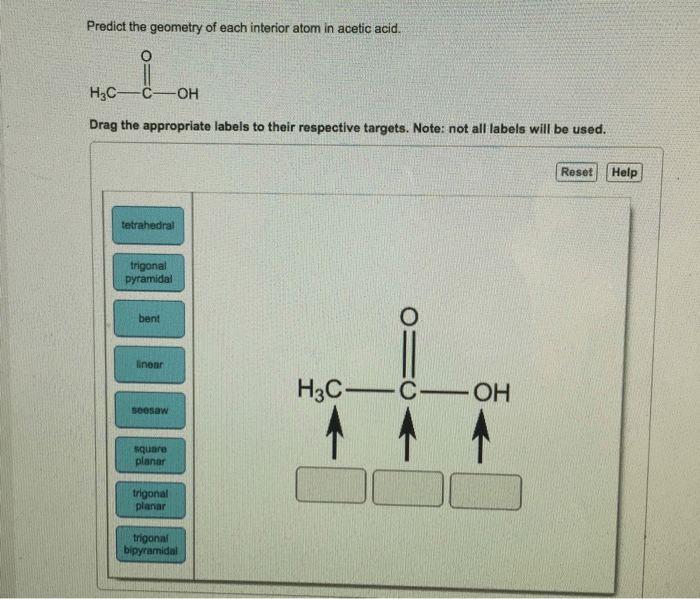
Acetic acid, with the molecular formula CH 3COOH, is a simple organic acid composed of a central carbon atom bonded to three other atoms: a hydrogen atom, an oxygen atom via a double bond, and a hydroxyl group (-OH). The central carbon atom is sp3hybridized, meaning it has four electron pairs arranged in a tetrahedral geometry.
The oxygen atoms in acetic acid have different bonding environments. The oxygen atom double-bonded to the central carbon is involved in a sp2hybrid orbital, resulting in a trigonal planar geometry. The oxygen atom single-bonded to the central carbon, part of the hydroxyl group, is sp3hybridized, leading to a tetrahedral electron-pair geometry.
Geometry Prediction of Interior Atoms: Predict The Geometry Of Each Interior Atom In Acetic Acid.
VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the molecular geometry of molecules based on the repulsion between electron pairs. According to VSEPR, electron pairs will arrange themselves in a way that minimizes repulsion, resulting in specific molecular shapes.
Electron-Pair Geometry and Molecular Geometry
The electron-pair geometry refers to the arrangement of electron pairs around an atom, while the molecular geometry considers both electron pairs and lone pairs. Lone pairs are electron pairs that are not involved in bonding and can influence the molecular geometry.
Oxygen Atom Double-Bonded to the Central Carbon
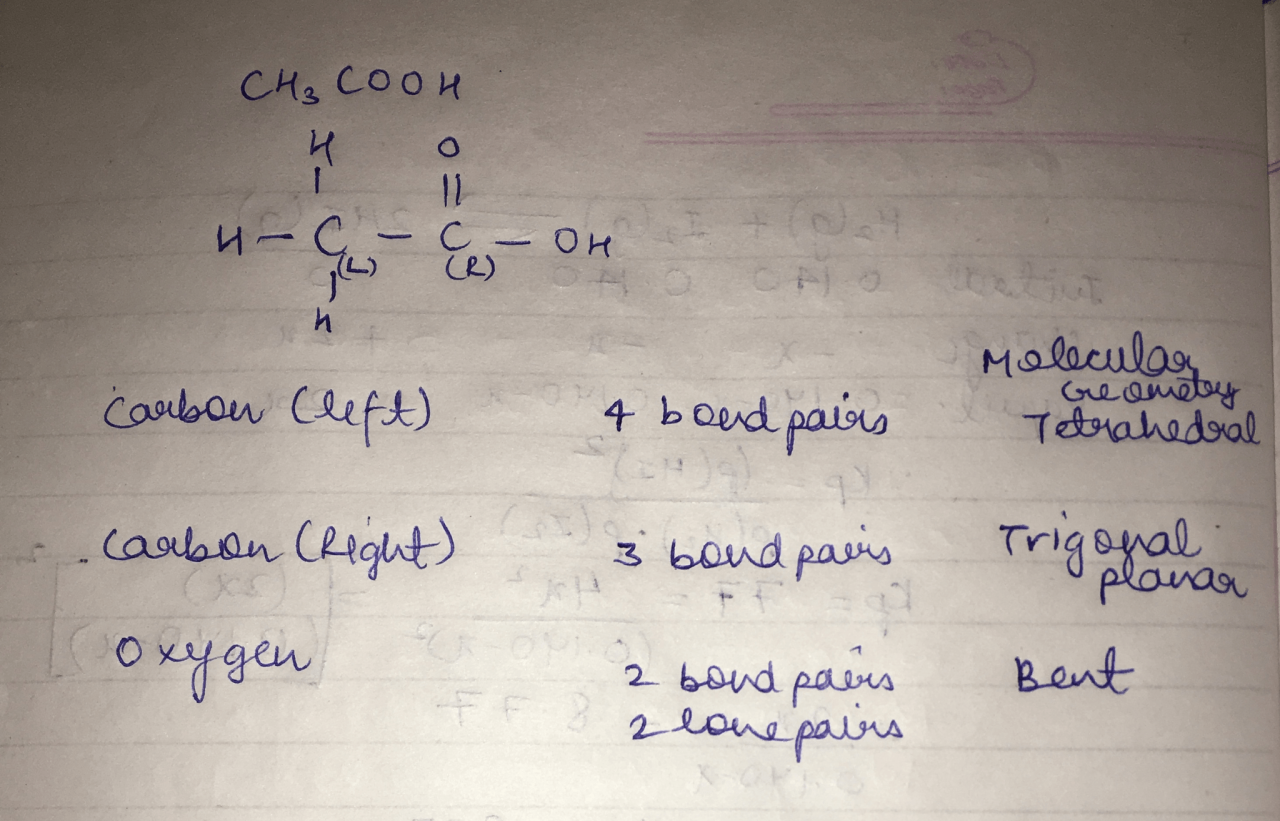
The oxygen atom double-bonded to the central carbon has two electron pairs: one involved in the double bond and two lone pairs. The VSEPR theory predicts a trigonal planar electron-pair geometry. Since there are no lone pairs, the molecular geometry is also trigonal planar.
Oxygen Atom Single-Bonded to the Central Carbon, Predict the geometry of each interior atom in acetic acid.
The oxygen atom single-bonded to the central carbon has four electron pairs: two involved in single bonds, one involved in a lone pair, and one involved in the partial double bond with the hydrogen atom in the hydroxyl group. The VSEPR theory predicts a tetrahedral electron-pair geometry.
Considering the lone pair, the molecular geometry is bent.
FAQ Corner
What is the molecular formula of acetic acid?
CH3COOH
How many oxygen atoms are bonded to the central carbon atom in acetic acid?
Two
What type of hybridization does the central carbon atom in acetic acid exhibit?
sp3